SUMMARY
WP1 covers the development and optimization of cutting-edge in vivo and in vitro models to assess gene therapies in lung transplantation. Building on existing in vivo and in vitro systems, WP1 develops novel approaches—including EVLP systems, organ-on-chip models, and isolated lung perfusion—to evaluate the efficacy of gene therapy interventions. These models support high-throughput, reproducible testing of gene-targeting strategies developed across the consortium and lay the groundwork for clinical translation.
WP1 leader is Prof. Clemens Aigner, from Medizinische Universität Wien.

Advanced mouse EVLP model for gene delivery
DC1 develops a novel ex vivo lung perfusion (EVLP) model in mice, aiming to maintain lung viability for up to 4 hours—crucial for evaluating genetic modification strategies. Using reporter mice, the model tests CRISPR-Cas9 and siRNA-based gene modulation in real time by tracking fluorescence changes. This scalable model allows for cost-effective, high-throughput testing of therapeutic approaches before proceeding to full LTx models.
Extended-duration EVLP with novel ventilation strategies
Classical EVLP involves positive pressure ventilation and a static prone position of the lung, both being associated with edema and inflammation that are correlated with worse outcomes and prevent prolonged EVLP. DC2 (HF) will assess the effectiveness of a new EVLP device “Revolution” from XVIVO which allows lung ventilation through negative pressure and continuous mobilization. They will evaluate the device’s capability to prolong stable EVLP up to 12 hours which would be favourable for any genetic manipulations. This new platform will be used in LifeLUNG for the first tests in human lungs declined for transplant.


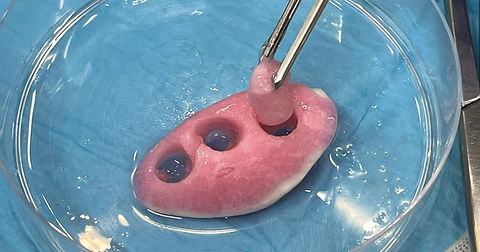
Isolated lobar perfusion in human lung tissue
DC3 develops an ex vivo isolated lobar perfusion model using human lung lobes obtained from thoracic surgery. This model permits targeted gene therapy testing on resected, diseased, or healthy lung tissue over prolonged perfusion. By evaluating immune and inflammatory responses, the model serves as a powerful tool for assessing GTA safety and efficacy in a clinically relevant context.
Organ-on-chip models to mimic IRI
DC4 constructs a microfluidic lung-on-chip platform that replicates ischemia-reperfusion injury (IRI) in a controlled environment. Human lung endothelial and stromal cells are cultured under dynamic perfusion, with immune cells introduced to simulate inflammatory responses. Oxygen deprivation and restoration mimic IRI events, allowing precise study of gene therapies' effects on lung microphysiology.

Integration with existing models and platforms
WP1 connects its new developments with pre-existing models—including porcine LTx, mouse rejection models, precision-cut lung slices, and ALI co-cultures—to enable iterative validation across systems. This synergy ensures robust testing pipelines for candidate gene therapies from WP2 and WP3, maximizing translational potential while minimizing animal use.


