SUMMARY
WP3 focuses on creating innovative tools for targeted gene delivery to lungs during ex vivo lung perfusion. These tools include lipid nanoparticles (LNPs), virus like particles and scalable biomanufacturing systems. By targeting inflammation and rejection pathways with siRNA, mRNA, and CRISPR-Cas systems, WP3 supports efficient, precise, and safe gene therapy interventions. The goal is to improve lung transplant success through personalized and scalable gene modulation strategies.
WP3 leader is Prof. Marianne Carlon, from KU Leuven.
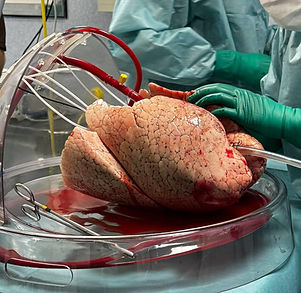
Targeted gene delivery during EVLP offers a therapeutic window
EVLP allows researchers to deliver gene therapy agents (GTAs) directly to the lung vasculature and airway epithelium before transplantation. WP3 develops methods to exploit this window, using airway or perfusate-based delivery of siRNA and CRISPR-Cas RNA cargo. These methods silence inflammatory genes and can modulate immune responses, reducing the risk of graft rejection and improving outcomes for lung transplant recipients.
Nanoparticle-based siRNA and mRNA delivery in lung models
University College London (UCL) (DC9) leads development of peptide-targeted lipid nanoparticles to deliver siRNA and mRNA into difficult-to-transfect lung tissues. These nanoparticles are tested in vitro using ALI cultures, lung organoids, and Lung-on-Chip models. The best formulations will then be validated in in vivo EVLP systems. Combined therapies delivering anti-inflammatory cytokines like TGF-β or IL-11 are also under investigation for added efficacy.
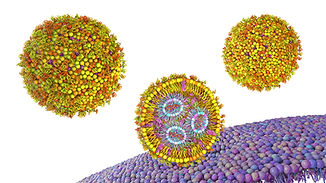
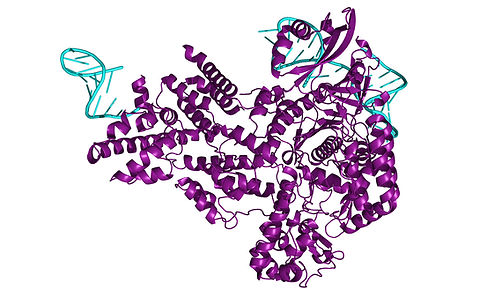
Cellular mechanisms affecting CRISPR/Cas delivery
DC10 (Aarhus Universitet - AU) is engineering CRISPR/Cas9 systems delivered via lentivirus-derived nanoparticles (LVNPs) to target genes involved in innate immunity and DNA damage response. These tools can knock out or suppress genes associated with ischemia-reperfusion injury (IRI) and primary graft dysfunction (PGD). Pseudotyped LVNPs, including those modified with SARS-CoV-2 spike proteins, enable precise delivery into ACE2+ lung endothelium.
Improving specificity with viral vectors and cell-type targeting
DC11 (KU Leuven - KUL) is mapping the cellular tropism of different viral delivery systems— in particular cell-derived vesicles (CDV) or virus like particles (VLP) —across lung cell types. They use single-cell RNA sequencing and next-gen sequencing to evaluate gene editing efficiency and immune responses. Custom ligands, nanobodies, and pseudotyping strategies are explored to enhance targeting of lung epithelial and endothelial cells, enabling precision interventions to reduce PGD.

Scaling up viral vector production for clinical application
To meet clinical demands, DC8 (KU Leuven - KUL / Antleron - ANT) is developing GMP-compliant manufacturing of viral vectors using fixed-bed bioreactor systems. This includes optimizing conditions like transfection timing, harvest processes, and flow dynamics using digital twins. A customized producer cell line will enhance yields for rAAVs and MLV-VLPs, balancing high throughput with cost-efficiency. This industrial-grade process supports the transition of therapies from lab to clinic.


